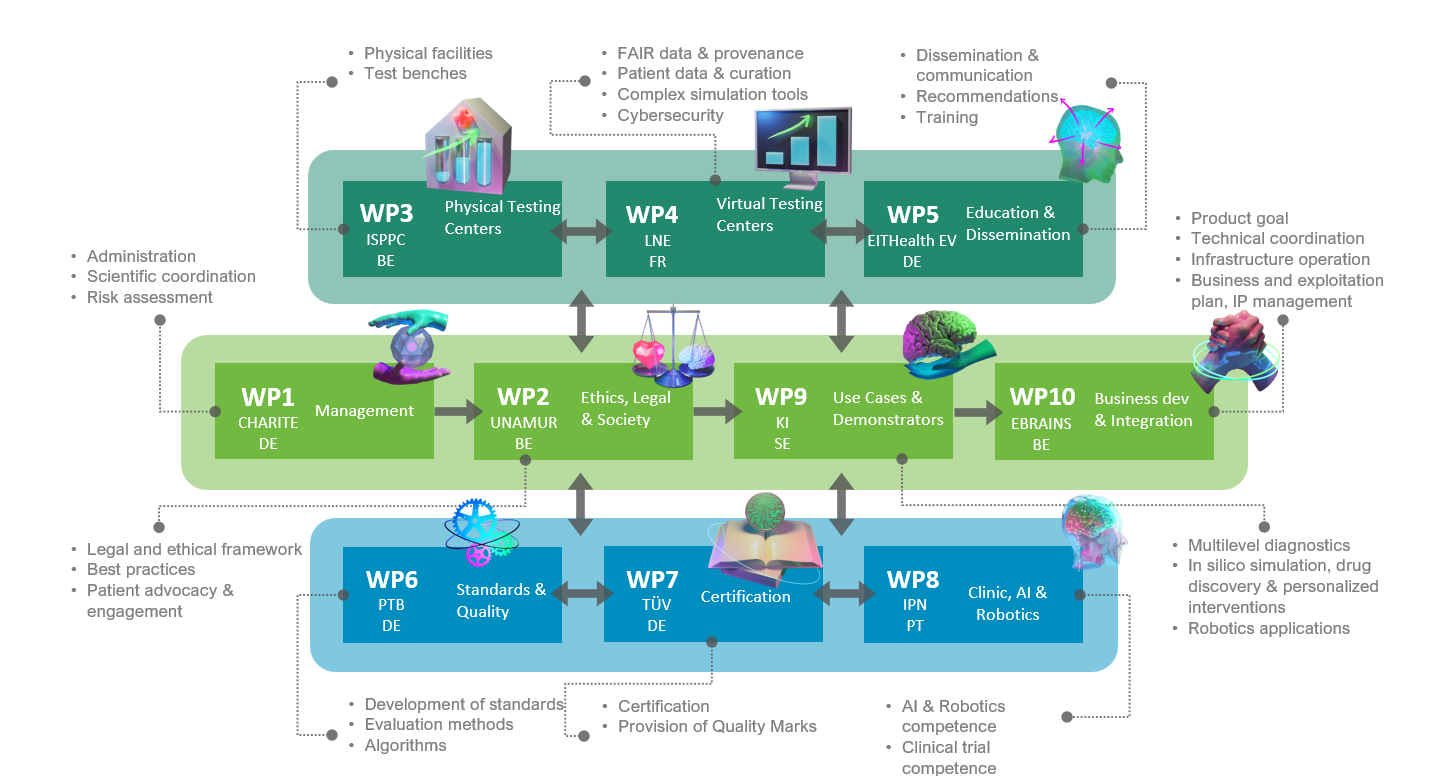
TEF-Health as a whole
To make the EU the place where AI excellence thrives from the lab to the market.
World-class Testing and Experimentation Facilities (TEFs) for AI.
Technological advances in the field of AI and robotics are being made at a breathtaking pace – and the healthcare sector is not spared from these developments. Yet it goes without saying that new medical devices and procedures must first prove their safety and usefulness before they can be adopted in clinical practice. In the European Union, the areas of AI and robotics, which are set to have a far-reaching impact on the healthcare sector, especially have to meet high quality requirements, but there is still a lack of testing infrastructure for developing standards, validating innovations, and certifying new products.
This is precisely where the Testing and Experimentation Facility for Health AI and Robotics (TEF-Health) comes in. The new project, supported by the EC and national funding agencies with a total of about €60 million, aims to “facilitate and accelerate the validation and certification of AI and robotics in medical devices,” explains Prof. Ritter, who coordinates the consortium and heads the Brain Simulation Section at the BIH. In total, 51 academic and private partners from nine European countries are involved in the project, integrating existing infrastructures as well as building new ones. The project started on 1 January 2023.
“With TEF-Health we mainly want to test novel AI approaches in real-world scenarios,” says Prof. Ritter. This includes new software used in areas such as patient care and diagnostics, as well as devices controlled by artificially intelligent programs, some of which are designed for direct use on humans – such as surgical and nursing robots. “We will evaluate how to facilitate market access and acceptance of these smart technologies,” Prof. Ritter reports.
Project partners plan to develop new regulatory and ethical requirements, including, for instance, standardized testing protocols and certifications or a specific code of conduct for use of the technology. In addition, the necessary technical and administrative procedures must be developed and created. Also on board the TEF-Health project are therefore leading hospitals, universities, and clinical research institutions such as Sweden’s Karolinska Institutet, as well as state-designated testing organizations such as German safety inspector TÜV and the National Metrology Institute of Germany and its French counterpart, the “Laboratoire national de métrologie et d'essais” (LNE). The newly created evaluation resources and infrastructure will be made available to industry in the future in the form of fee-based services. “Widespread use of these comprehensive testing and evaluation tools will not only accelerate market access for innovative AI and robotics technologies, but will also ultimately boost public confidence in these new developments,” explains Prof. Ritter.
The TEF-Health project has the express aim of generating and consolidating sustainable collaborations between industry, academic research, and other players. “Long-term partnerships in innovation networks have shown to provide a particularly favorable environment for the transfer of technology from research to practice,” Prof. Ritter explains. “What’s more, close cooperation between the various partners avoids duplicating work already done.” This, she says, makes sure investments are used for highest impact. The close collaboration will also help to ensure that going forward research findings are translated more quickly into new products and services. Ultimately, the entire value chain in AI and robotics technologies for healthcare will benefit from this – which in turn will “increase the prosperity and quality of life of society as a whole,” predicts Prof. Ritter.
So in the end, TEF-Health will contribute to Digital Europe’s overall aim of increasing the effectiveness, resilience, and sustainability of European health and care systems and reducing healthcare delivery inequalities in Europe, while ensuring compliance with relevant legal, ethical, quality, and interoperability standards and requirements.